|
Acetic anhydride
CAS number 108-24-7
Product Identification
Synonyms: Acetyl oxide; Acetic acid anhydride; Acetic oxide; Ethanoic
anhydride
CAS No.: 108-24-7
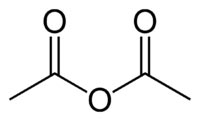
Molecular Weight: 102.09
Chemical Formula: (CH3CO)2O
Product Codes:
J.T. Baker: 0018
Mallinckrodt: 2420
Hazards Identification
DANGER! CORROSIVE. CAUSES BURNS TO ANY AREA OF CONTACT. FLAMMABLE LIQUID
AND VAPOR. WATER REACTIVE. HARMFUL IF SWALLOWED OR INHALED. VAPOR CAUSES
RESPIRATORY TRACT IRRITATION AND SEVERE EYE IRRITATION.
SAF-T-DATA(tm) Ratings (Provided here for your convenience) Health
Rating: 3 - Severe
Flammability Rating: 2 - Moderate
Reactivity Rating: 2 - Moderate
Contact Rating: 4 - Extreme (Corrosive)
Lab Protective Equip: GOGGLES & SHIELD; LAB COAT & APRON; VENT HOOD;
PROPER GLOVES; CLASS B EXTINGUISHER
Storage Color Code: Red Stripe (Store Separately) Potential Health
Effects
Inhalation:
Vapors are corrosive to the mucous membranes of the upper respiratory
tract. Exposure to vapors may cause irritation of the nose, throat, and
coughing. Exposure to high concentrations may result in severe damage to
the lungs. Symptoms of lung edema are often delayed and are aggravated
by physical effort.
Ingestion:
Corrosive. Causes a burning pain in the stomach, followed by nausea and
vomiting.
Skin Contact:
Corrosive: Does not cause severe burning on contact but can cause
delayed reaction burns. If not removed by washing, the skin may become
reddened and later turn white and wrinkled. Continued skin contact may
cause dermatitis.
Eye Contact:
Corrosive: Contact with the liquid or vapor may produce a burning
sensation and tearing. Redness, pain and blurred vision may be followed
by permanent eye damage. The appearance of eye burns may be delayed.
Irritation effects begin with airborne concentrations as low as 0.36
mg/m3.
Chronic Exposure:
Repeated and prolonged exposure to vapor may cause irritation of the
skin and chronic eye irritation.
Aggravation of Pre-existing Conditions:
Persons with pre-existing skin disorders or eye problems, or impaired
respiratory function may be more susceptible to the effects of the
substance. Fire:
Flash point: 49C (120F) CC
Autoignition temperature: 316C (601F)
Flammable limits in air % by volume:
lel: 2.7; uel: 10.3
Flammable.
Explosion:
Above flash point, vapor-air mixtures are explosive within flammable
limits noted above. Sealed containers may rupture when heated. Vapors
can flow along surfaces to distant ignition source and flash back. A
violent exothermic reaction occurs with water. Sufficient heat may be
produced to ignite combustible materials. Sensitive to static discharge.
Fire Extinguishing Media:
Water spray, dry chemical, alcohol foam, or carbon dioxide. Use water
with caution as material reacts with water.
Special Information:
In the event of a fire, wear full protective clothing and NIOSH-approved
self-contained breathing apparatus with full facepiece operated in the
pressure demand or other positive pressure mode. Use water spray to
blanket fire, cool fire exposed containers, and to flush non-ignited
spills or vapors away from fire.
Handling and Storage
Protect against physical damage. Store in a cool, dry well-ventilated
location, away from any area where the fire hazard may be acute. Outside
or detached storage is preferred. Separate from incompatibles.
Containers should be bonded and grounded for transfers to avoid static
sparks. Storage and use areas should be No Smoking areas. Use
non-sparking type tools and equipment, including explosion proof
ventilation. Keep away from water. This material is corrosive to steel,
galvanized iron, copper and copper alloys. Containers of this material
may be hazardous when empty since they retain product residues (vapors,
liquid); observe all warnings and precautions listed for the product.
Physical and Chemical Properties
Appearance: Clear, colorless liquid.
Odor:Strong acetic odor; good warning properties.
Solubility:Slowly soluble in water (reacts)
Specific Gravity:1.08 @ 15C/4C
pH:No information found.
% Volatiles by volume @ 21C (70F):100
Boiling Point:140C (284F)
Melting Point:-73C (-99F)
Vapor Density (Air=1):3.52
Vapor Pressure (mm Hg):4 @ 20C (68F)
Evaporation Rate (BuAc=1):0.46
Other Information
NFPA Ratings: Health: 3 Flammability: 2 Reactivity: 1
Label Hazard Warning:
DANGER! CORROSIVE. CAUSES BURNS TO ANY AREA OF CONTACT. FLAMMABLE LIQUID
AND VAPOR. WATER REACTIVE. HARMFUL IF SWALLOWED OR INHALED. VAPOR CAUSES
RESPIRATORY TRACT IRRITATION AND SEVERE EYE IRRITATION.
Label Precautions:
Do not get in eyes, on skin, or on clothing.
Do not contact with water.
Do not breathe vapor.
Keep container closed.
Use only with adequate ventilation.
Wash thoroughly after handling.
Keep away from heat, sparks and flame.
Label First Aid:
In case of contact, immediately flush eyes or skin with plenty of water
for at least 15 minutes while removing contaminated clothing and shoes.
Wash clothing before reuse. If inhaled, remove to fresh air. If not
breathing, give artificial respiration. If breathing is difficult, give
oxygen. If swallowed, DO NOT INDUCE VOMITING. Give large quantities of
water. Never give anything by mouth to an unconscious person. In all
cases get medical attention immediately.
Product Use:
Laboratory Reagent.
| |
|
Note /Government
Notification: These chemicals are designated as those that are used
in the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT.
Information: The information on this web page is provided to help
you to work safely, but it is intended to be an overview of hazards,
not a replacement for a full Material Safety Data Sheet (MSDS). MSDS
forms can be downloaded from the web sites of many chemical
suppliers. ,also that the information on the PTCL Safety web site,
where this page was hosted, has been copied onto many other sites,
often without permission. If you have any doubts about the veracity
of the information that you are viewing, or have any queries, please
check the URL that your web browser displays for this page. If the
URL begins "www.tajapi.com/www/Denatonium Benzoate.htm/" the page is
maintained by the Safety Officer in Physical Chemistry at Oxford
University. If not, this page is a copy made by some other person
and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive Drug
Abuse Prevention and Control Act of 1970.[1] The CSA is the federal
U.S. drug policy under which the manufacture, importation,
possession, use and distribution of certain substances is regulated.
The Act also served as the national implementing legislation for the
Single Convention on Narcotic Drugs |
|
|
|
|








